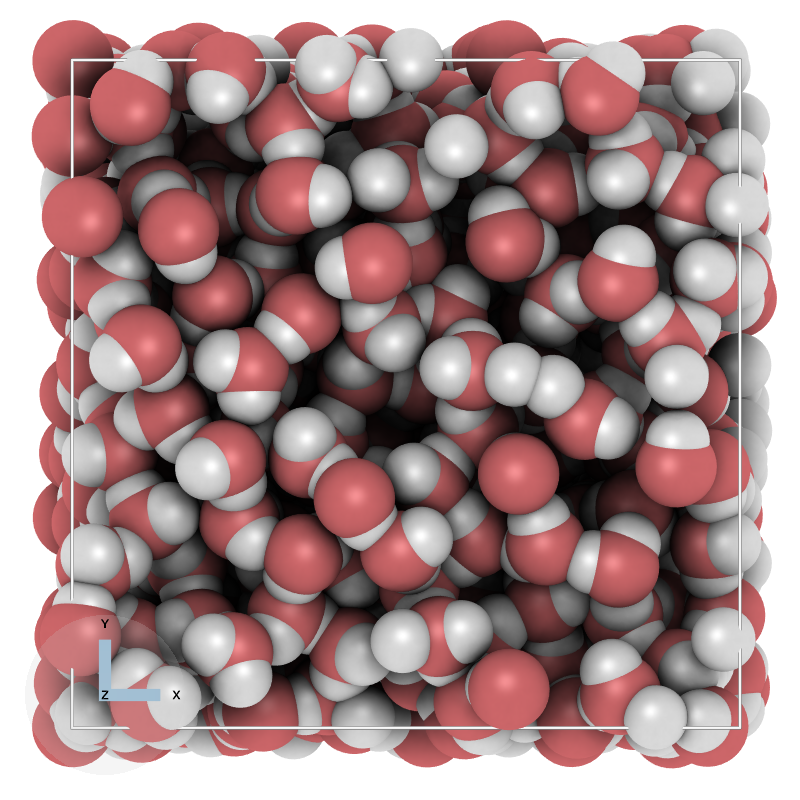
Classical water-model (SPC, SPE, Tip3p) are based on placing fixed point-charges at the water atoms (oxygen and hydrogens), or for more advanced model like (Tip4p, Tip5p) also at the positions of the lone-pairs. The idea of the later models to better described the charge distribution around the water molecules. In addition to the charges, the models have Lennard-Jones potentials describing the Van der Waals interactions of the water with other water molecules and the environment.
A common way of obtaining the Lennard-Jones parameters is to use experimental Vapor-Liquid Equilibrium (VLE) data and fit the parameters to the experimental data. However, common classical water-models are not able to reproduce both the liquid phase and the vapor phase. The main culprit is the fixed-charges assumption. Placing fixed point charges means a fixed dipole-moment. From experiments it is well-known that the dipole-moment of the liquid-phase is very different from the dipole moment of water in the gas-phase.
In this computer simulation project, we are going to solve this problem and develop a classical water model that is applicable to both the liquid-phase and also the vapor-phase of water.